

AI-Powered Clinical Trial Monitoring
Automate scheduling, optimize routes, and streamline site oversight
Automate & Streamline Monitoring Visit Planning
SiteIQ360 is an AI scheduling and optimization platform that automates and streamlines monitoring visit planning for CRAs and Clinical Operations teams.
AI that makes site oversight smarter and faster

Predictive Scheduling
AI learns from CRA behavior and study data
Route Optimization
Minimize travel time and maximize efficiency
Dynamic Rescheduling
Adapt to changes in real-time
Our Mission
To revolutionize clinical trial operations with AI-powered scheduling and monitoring automation, ensuring faster, more efficient, and compliant site oversight.
Powerful Features for Clinical Research
Everything you need to streamline clinical trial monitoring and accelerate research outcomes.

Meet Atria: Your AI-Powered CRA Virtual Assistant
Atria is the intelligent chatbot at the heart of SiteIQ360, designed specifically for Clinical Research Associates. Powered by advanced AI, Atria understands your workflow, anticipates your needs, and helps you make smarter scheduling decisions in real-time.
Whether you need to reschedule a visit, optimize your route, or get insights on site performance, Atria is always ready to assist with natural, conversational interactions.

Natural Language Interface
Ask Atria questions in plain English and get instant, intelligent responses about your schedule.
Smart Scheduling Assistant
Atria proactively suggests optimal visit times and helps you manage complex multi-site schedules.
Instant Insights
Get real-time analytics and recommendations to improve your monitoring efficiency.
Predictive Guidance
Receive AI-powered forecasts and alerts about potential scheduling conflicts or delays.
How SiteIQ360 Works
Five simple steps to transform your clinical trial monitoring process.
"We integrate with existing CTMS tools, learn from operational data, and generate optimized visit plans — fully compliant with ICH-GCP."
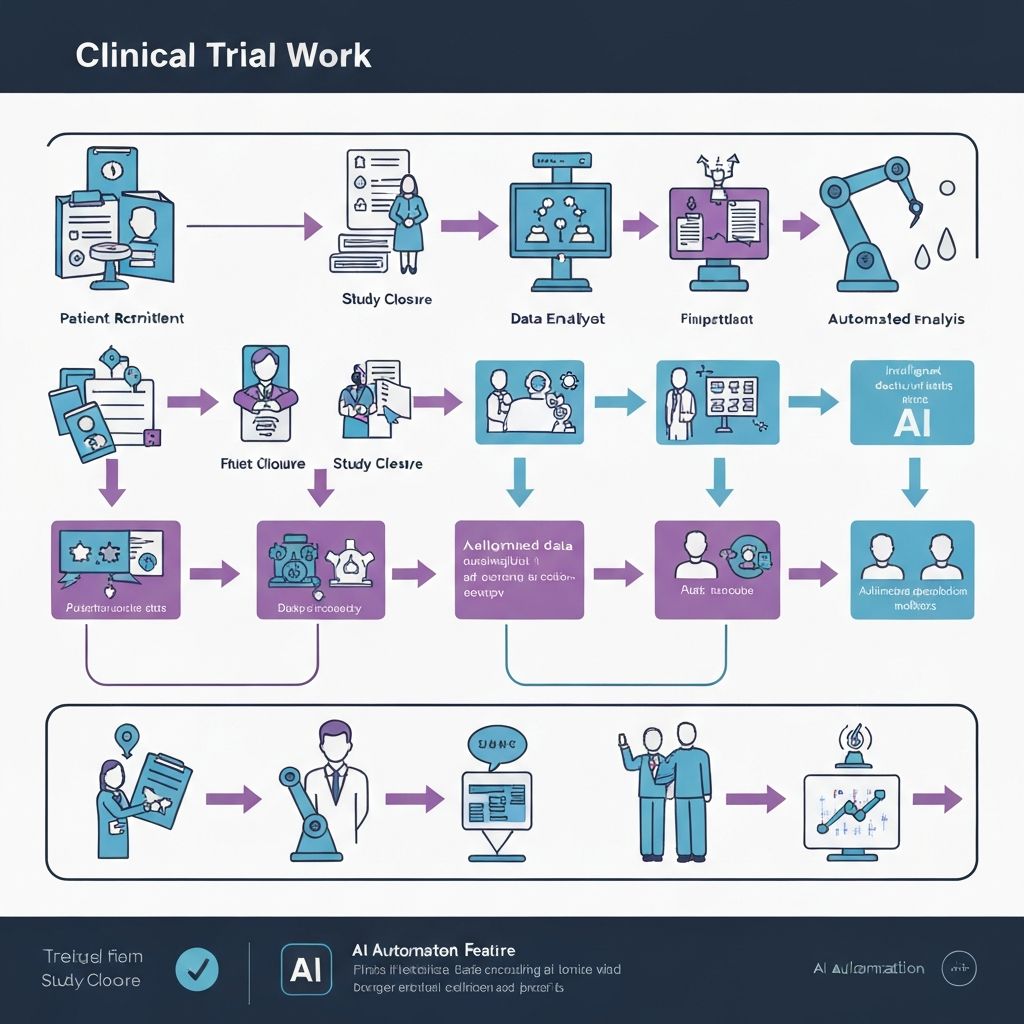
Data from CTMS / EDC / Trial Systems
Connect with your existing Clinical Trial Management Systems, Electronic Data Capture platforms, and trial databases to import study protocols and site information.
AI Engine Analyzes Risk & Timelines
Our AI engine powered by Atria analyzes historical patterns, risk factors, protocol timelines, and CRA availability to identify optimal scheduling opportunities.
Optimized Visit Plan
Receive intelligent visit schedules with optimized routes, automated conflict resolution, and full ICH-GCP compliance checks built in.
CRA Confirmation
CRAs review and confirm the AI-generated visit plans through an intuitive interface, with the ability to make adjustments as needed.
Automatic Updates Back to Sponsor
Confirmed schedules are automatically synchronized back to sponsor systems, ensuring all stakeholders have real-time visibility.
Powerful Analytics & Insights
Real-time dashboards that give you complete visibility into your clinical trial operations
Visit Coverage Analytics
Track visit completion rates, identify gaps, and ensure comprehensive site oversight across all trials
Missed Visits Tracking
Instantly identify and address missed or delayed visits to maintain protocol compliance
CRA Utilization Metrics
Monitor workload distribution, productivity, and capacity across your CRA team
Travel Analytics & Route Maps
Visualize CRA travel patterns, optimize routes, and reduce travel time and costs
Scheduled Visits Overview
View upcoming visits, manage schedules, and ensure optimal site coverage planning
Performance Trends
Track key performance indicators and identify opportunities for operational improvement

Compliance-First Approach
Built with regulatory compliance at its core, ensuring your clinical trials meet the highest standards.
GCP Compliant
Fully aligned with Good Clinical Practice guidelines and ICH-GCP standards.
GDPR Protected
Complete data protection compliance with EU General Data Protection Regulation.
Audit Trail
Comprehensive audit logs for all scheduling decisions and system actions.
Global Standards
Meets international regulatory requirements for clinical trial management.
About the Founder
Built by a CRA, for CRAs
After working as a Clinical Research Associate (CRA) for more than a decade, I've seen how our work plays a vital role in advancing medicine and improving patient outcomes. Being a CRA isn't just a job — it's a commitment to quality, precision, and patient safety. But over the years, I also experienced the practical challenges that come with the role.
One of the biggest challenges I faced was managing multiple site visits each month — from booking and rescheduling visits to keeping track of them all. These tasks often consumed a lot of time and mental space. In addition, ensuring visit compliance required constant attention and manual checks, which could be both time-consuming and stressful.
I realised that while the clinical research industry has made huge advances, the tools available to CRAs haven't evolved as quickly. Too much of our time is still spent on administrative work instead of focusing on what really matters — conducting high-quality monitoring and supporting sites and patients.
That's what inspired me to create SiteIQ360 — a platform built by a CRA, for CRAs.
SiteIQ360 is designed to simplify the way we plan, manage, and track our monitoring visits. It aims to make our workflows more efficient, reduce time spent on repetitive tasks, and help CRAs focus on delivering excellence in clinical research.
My goal is simple: to empower CRAs with smart, intuitive tools that make our jobs easier and more impactful — and to drive clinical research forward with efficiency and integrity at its core.
Thank you for joining me on this journey. Together, we can redefine what it means to work smarter in clinical research — one site, one visit, one study at a time.
Syed Arafath
Founder, SiteIQ360
Ready to Transform Your Clinical Trial Monitoring?
Join leading pharmaceutical companies and CROs who are accelerating clinical research with AI-powered scheduling.